Article by Will Fuller
Published: 23 February 2023
New Study out by Dr Will Fuller in PNAS News where the years of work finally unveils the story of how #palmitoylation of the pore-forming alpha subunit regulates the voltage sensitive Ca channel Ca(v)1.2 in the heart
Palmitoylation of the pore-forming subunit of Ca(v)1.2 controls channel voltage sensitivity and calcium transients in cardiac myocytes
Chien-Wen S. Kuoa, Sara Dobia, Caglar Göka, Ana Da Silva Costaa, Alice Maina, Olivia Robertson-Graya, Daniel Baptista-Honb,c, Krzysztof J. Wypijewskia, Hannah Costellob, Tim G. Halesb, Niall MacQuaidea, Godfrey L. Smitha, and William Fuller
Abstract
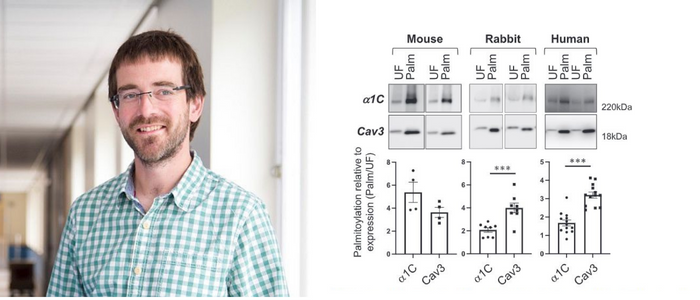
Mammalian voltage-activated L-type Ca2+ channels, such as Ca(v)1.2, control transmem-brane Ca2+ fluxes in numerous excitable tissues. Here, we report that the pore-forming α1C subunit of Ca(v)1.2 is reversibly palmitoylated in rat, rabbit, and human ventricular myocytes. We map the palmitoylation sites to two regions of the channel: The N termi-nus and the linker between domains I and II. Whole-cell voltage clamping revealed a rightward shift of the Ca(v)1.2 current–voltage relationship when α1C was not palmi-toylated. To examine function, we expressed dihydropyridine-resistant α1C in human induced pluripotent stem cell-derived cardiomyocytes and measured Ca2+ transients in the presence of nifedipine to block the endogenous channels. The transients generated by unpalmitoylatable channels displayed a similar activation time course but signifi-cantly reduced amplitude compared to those generated by wild-type channels. We thus conclude that palmitoylation controls the voltage sensitivity of Ca(v)1.2. Given that the identified Ca(v)1.2 palmitoylation sites are also conserved in most Ca(v)1 isoforms, we propose that palmitoylation of the pore-forming α1C subunit provides a means to regulate the voltage sensitivity of voltage-activated Ca2+ channels in excitable cells.
Voltage-activated channels facilitate the movement of ions across the membranes of excit-able tissues in response to changes in membrane potential. The L-type Ca2+ channel mediates the depolarization-induced entry of Ca2+ into numerous cell types, thereby controlling excitation–contraction coupling in smooth and striated muscles, excitation–secretion coupling in endocrine cells, and neurotransmitter release in neurons (1). The precise control of L-type Ca2+ channel activity is therefore important in a diverse range of physiological settings—from the contraction of cardiac muscle in the control of cardiac output to the contraction of blood vessels in the control of blood pressure and the secretion of insulin in glucose homeostasis.Voltage-activated Ca2+ channels are composed of multiple subunits, including a pore-forming α subunit and an accessory β subunit (2). The α subunit (α1C in the cardiac L-type Ca2+ channel Ca(v)1.2) is a transmembrane protein that has four domains (I to IV, each with six membrane-spanning units), which are connected by intracellular loops.
The β subunit is cytosolic, interacts with the intracellular loop between domains I and II of the α subunit, and regulates numerous aspects of channel behavior (3).There are four subfamilies of β subunits (β1 to β4) encoded by distinct genes which can be alternatively spliced (4). Specifically, β subunits enhance Ca(v)1.2-mediated cur-rents by promoting channel exit from the endoplasmic reticulum (4). Once Ca(v)1.2 is at the cell surface, β subunits promote channel activation by shifting the voltage depend-ence of opening to more hyperpolarized potentials and accelerating channel activation (5, 6). Two forms of activity-dependent inactivation control most voltage-sensitive Ca2+channels: Voltage-dependent inactivation (VDI) and calcium-dependent inactivation (CDI). Both VDI and CDI contribute to regulation of neuronal P/Q-type (Ca(v)2.1) Ca2+ channels, (7, 8) whereas CDI usually dominates for L-type channels such as Ca(v)1.2 in the heart (8–10). Most β subunits enhance both VDI and CDI, but β2a specifically reduces VDI.
First published: 23 February 2023

