SCMH Staff lead paper recently published in the Lancet Diabetes and Endocrinology.
Published: 1 September 2021
Prof Naveed Sattar, Dr Matthew Lee (as joint first authors) and Porf John McMurray co-authored a new meta-analysis on the cardiovascular and renal effects of GLP-1RA class of drugs used in diabetes, in the Lancet Diabetes and Endocrinology
Profs Naveed Sattar, Matthew Lee (as joint first authors) and John McMurray co-authored a new meta-analysis on the cardiovascular and renal effects of GLP-1RA class of drugs used in diabetes, in the Lancet Diabetes and Endocrinology journal.
'Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials'
Prof Naveed Sattar MDa†Matthew M YLee MBChBa†Søren L Kristensen MDb†Prof Kelley R HBranch MDc Prof Stefano Del PratoMDd Nardev S KhurmiMDeProf Carolyn S PLam MBBSfProf Renato D LopesMDgProf John J V McMurray MDaProf Richard E Pratley MDhProf Julio RosenstockMDiProf Hertzel C GersteinMDj
Link to paper: sciencedirect.com
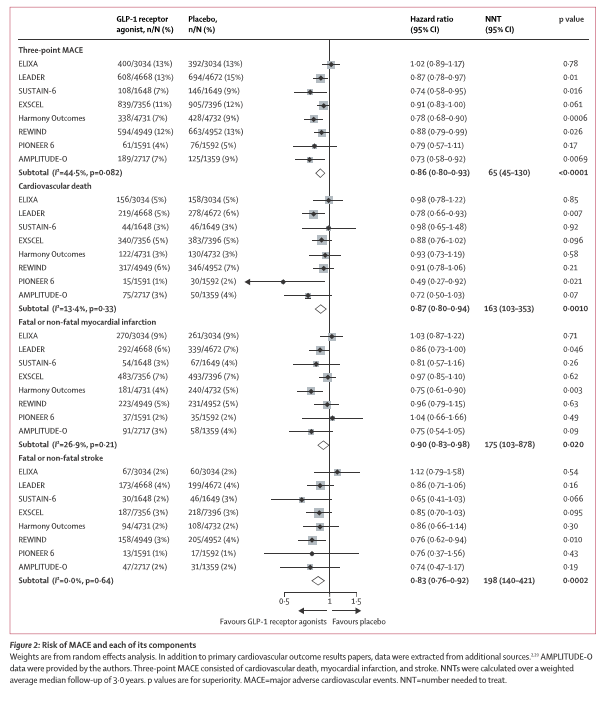
*See figure below
BACKGROUND
GLP-1 receptor agonists reduce major adverse cardiovascular events (MACE) in patients with type 2 diabetes. However, uncertainty regarding kidney outcomes persists and whether benefits extend to exendin-4-based GLP-1 receptor remains uncertain. We aimed to meta-analyse the most up-to-date evidence on the cardiovascular benefits and risks of GLP-1 receptor agonists from outcome trials in patients with type 2 diabetes.
FINDINGS
Of 98 articles screened, eight trials comprising 60 080 patients fulfilled the prespecified criteria and were included. Overall, GLP-1 receptor agonists reduced MACE by 14% (HR 0·86 [95% CI 0·80–0·93]; p<0·0001), with no significant heterogeneity across GLP-1 receptor agonist structural homology or eight other examined subgroups (all pinteraction≥0·14). GLP-1 receptor agonists reduced all-cause mortality by 12% (HR 0·88 [95% CI 0·82–0·94]; p=0·0001), hospital admission for heart failure by 11% (HR 0·89 [95% CI 0·82–0·98]; p=0·013), and the composite kidney outcome by 21% (HR 0·79 [95% CI 0·73–0·87]; p<0·0001), with no increase in risk of severe hypoglycaemia, retinopathy, or pancreatic adverse effects. In sensitivity analyses removing the only trial restricted to patients with an acute coronary syndrome (ELIXA), all benefits marginally increased, including the outcome of worsening of kidney function, based on eGFR change (HR 0·82 [95% CI 0·69–0·98]; p=0·030).
INTERPRETATION
GLP-1 receptor agonists, regardless of structural homology, reduced the risk of individual MACE components, all-cause mortality, hospital admission for heart failure, and worsening kidney function in patients with type 2 diabetes.
This new meta-analysis includes data from a recently reported diabetes outcome trial, AMPLITUDE-O published in the NEJM
'Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes'
Hertzel C. Gerstein, M.D., Naveed Sattar, M.D., Ph.D., Julio Rosenstock, M.D., Chinthanie Ramasundarahettige, M.Sc., Richard Pratley, M.D., Renato D. Lopes, M.D., Ph.D., Carolyn S.P. Lam, M.B., B.S., Ph.D., Nardev S. Khurmi, M.D., Laura Heenan, M.Sc., Stefano Del Prato, M.D., Leanne Dyal, M.Sc., and Kelley Branch, M.D. for the AMPLITUDE-O Trial Investigators
BACKGROUND
Four glucagon-like peptide-1 (GLP-1) receptor agonists that are structurally similar to human GLP-1 have been shown to reduce the risk of adverse cardiovascular events among persons with type 2 diabetes. The effect of an exendin-based GLP-1 receptor agonist, efpeglenatide, on cardiovascular and renal outcomes in patients with type 2 diabetes who are also at high risk for adverse cardiovascular events is uncertain.
METHODS
In this randomized, placebo-controlled trial conducted at 344 sites across 28 countries, we evaluated efpeglenatide in participants with type 2 diabetes and either a history of cardiovascular disease or current kidney disease (defined as an estimated glomerular filtration rate of 25.0 to 59.9 ml per minute per 1.73 m2 of body-surface area) plus at least one other cardiovascular risk factor. Participants were randomly assigned in a 1:1:1 ratio to receive weekly subcutaneous injections of efpeglenatide at a dose of 4 or 6 mg or placebo. Randomization was stratified according to use of sodium–glucose cotransporter 2 inhibitors. The primary outcome was the first major adverse cardiovascular event (MACE; a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or undetermined causes).
CONCLUSIONS
In this trial involving participants with type 2 diabetes who had either a history of cardiovascular disease or current kidney disease plus at least one other cardiovascular risk factor, the risk of cardiovascular events was lower among those who received weekly subcutaneous injections of efpeglenatide at a dose of 4 or 6 mg than among those who received placebo. (Funded by Sanofi; AMPLITUDE-O ClinicalTrials.gov number, NCT03496298. opens in new tab.)
Prof Naveed Sattar was on the steering committee. These two papers add support for the cardiovascular benefits of this class of drugs, potentially strongest for prevention of stroke (risk reduced by 17-19%). They also add support for GLP-1 RA’s reducing the risk of heart failure (11%), albeit modestly, and for their kidney benefits extending to harder kidney outcomes (reduced by 18% in sensitivity analyses). Such data will help inform future guidelines and the design of future trials on this class of drugs.
First published: 1 September 2021

