New Paper led by Chloe Gulliver
Published: 2 October 2023
Paper from Baillie Lab published in British Journal of Cancer
Loss of PDE4D7 expression promotes androgen independence, neuroendocrine differentiation and alterations in DNA repair: implications for therapeutic strategies
Chloe Gulliver, Sebastian Huss, Axel Semjonow, George S Baillie, Ralf Hoffmann
Summary
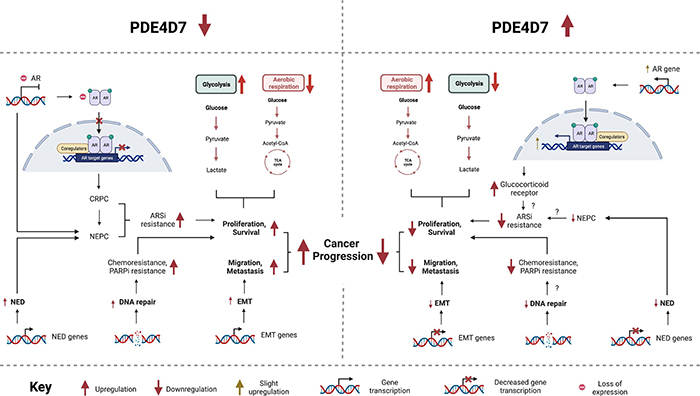
PDE4D7 expression diminishes throughout prostate cancer (PCa) progression, with low expression conferring an aggressive disease phenotype. Using stable shRNA-mediated knockdown of PDE4D7 in LNCaP cells, we replicated clinical observations and phenocopied the transition from androgen-sensitive to androgen-insensitive PCa.
Using next generation RNA sequencing and gene set enrichment analysis, key molecular pathways implicated upon PDE4D7 knockdown were identified, such as the androgen response pathway. Decreased PDE4D7 resulted in loss of the androgen receptor (AR), coinciding with resistance to androgen receptor signalling inhibition via enzalutamide. Furthermore, DNA repair pathways were affected by knockdown of PDE4D7 expression, which conferred resistance to chemotherapy and PARP inhibition, highlighting PDE4D7's role in mediating the DNA damage response, with PDE4D7 loss conferring enhanced ability for PCa cells to repair DNA damage and evade apoptosis.
Depletion of PDE4D7 also promoted the enrichment of pathways associated with development of an aggressive phenotype, such as epithelial-mesenchymal transition (EMT) and neuroendocrine differentiation (NED), overall implicating PDE4D7 loss in the development of the most aggressive form of PCa, neuroendocrine prostate cancer (NEPC), characterised by AR-negativity, NED, enhanced proliferation and therapeutic resistance. Our findings further revealed that re-introduction of PDE4D7 led to slight restoration of AR and AR-target gene expression and, alongside pharmacological PDE4 activation, re-sensitised PDE4D7 knockdown LNCaPs to enzalutamide.
Overall, augmentation of PDE4D7 expression and/or activity is capable of hindering aggressive growth characteristics as well as restoring therapeutic sensitivity in resistant tumours, ultimately representing a novel therapeutic strategy for patients with high-risk CRPC and NEPC for whom today treatment options are extremely limited.
First published: 2 October 2023

