Short PDE4 Isoforms as Drug Targets in Disease
Published: 31 July 2023
New paper led by Elka Kyurkchieva
Short PDE4 Isoforms as Drug Targets in Disease
Elka Kyurkchieva, George S Baillie
Summary
The second messenger, cyclic adenosine monophosphate (cAMP), is a master regulator of signal transduction that maintains cell homeostasis. The balance between cAMP synthesis by adenylyl cyclase and degradation by phosphodiesterases (PDEs) underpins receptor-specific responses. As multiple receptors rely on cAMP for signalling, PDEs shape three-dimensional, localised gradients of the cyclic nucleotide to drive appropriate signalling cascades. The PDE4 family, which comprises long, short, and supershort isoforms and a dead-short isoform, is of great interest due to its implication in disease. While long PDE4 isoforms are in the research spotlight as drug targets, short and supershort isoforms (SASSI) remain less well studied.
Our review provides a record of the discovery, regulation, and disease relevance of PDE4 SASSI. An overarching theme is their role in immune cell activation, which makes them attractive drug targets in multiple sclerosis, Alzheimer’s disease, cancer, and chronic obstructive pulmonary disease. Briefly, upregulation of PDE4 SASSI has been reported to exacerbate the immune response in inflammatory diseases and lead to increased cell proliferation in malignancy. Despite continued research efforts, selective downregulation of disease-causing PDE4 isoforms remains an obstacle: clinically approved small molecule inhibitors targeting the catalytic site are limited by severe side effects owing to the high degree of conservation of the catalytic domain between over 20 unique PDE4 isoenzymes. Experimental compounds exhibiting partial isoform selectivity are exclusively aimed at long PDE4 isoforms. Resultingly, PDE4 SASSI represent an underexploited target for specific inhibition. Utilising the structural differences between long and short PDE4 isoforms to design novel inhibitors may contribute to overcoming the challenge of isoform-selective PDE4 inhibition and improve patient quality of life.
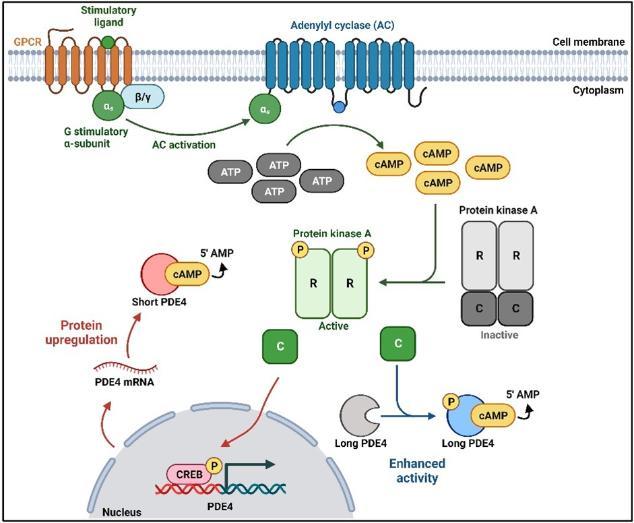
Long and short PDE4 regulation. G-protein-coupled receptor (GPCR) activation induces adenylyl cyclase (AC) activation and the production of cyclic adenosine monophosphate (cAMP) from adenosine triphosphate (ATP). Increased cAMP levels lead to phosphorylation and activation of Protein kinase A (PKA). Conformational changes induced by the phosphorylation of PKA lead to the dissociation of the PKA catalytic (C) subunit from the regulatory (R) subunit dimer. The catalytic subunit can phosphorylate long PDE4 isoforms to enhance their activity and phosphorylate the cAMP-response element binding (CREB) protein to enhance short, supershort, and dead-short PDE4 isoform transcription, which in turn drives upregulated protein expression. Created with BioRender.com.
First published: 31 July 2023

