Ethics approval for veterinary and animal related research projects
All veterinary and animal related research projects must have ethics approval prior to starting. This includes research on all vertebrates and certain invertebrates ie decapod crustaceans or cephalopod molluscs. Depending on the type of research, one or more of the following committees may need to be consulted:
- AWERB Committee (Enquiries email: biologicalservices-aspa-enquiries@lists.cent.gla.ac.uk) (Research involving animals that may involve pain distress, suffering or lasting harm)
Projects that ‘cause pain distress, suffering or lasting harm to an animal for research purposes only’ fall under the Animal (Scientific Procedures) Act 1986 (ASPA), and ethics approval is obtained from the Animal Welfare and Ethics Research Body (AWERB). For work that would fall under ASPA if conducted in the UK but is being conducted overseas, there is a new sub-committee of AWERB. For any projects that might fall under ASPA (UK or elsewhere), contact one of the Named Veterinary Surgeons in the first instance.
- SBOHVM Ethics Committee (Email a Research Ethics Application Form to SBOHVM-ethics-requests@glasgow.ac.uk) (Research involving animals that does not fall under ASPA (including animal data, biological samples))
The School of Biodiversity, One Health and Veterinary Medicine (SBOHVM) Research Ethics and Welfare Committee will consider applications for all prospective non ASPA studies and retrospective analyses concerning all veterinary species, including questionnaire studies concerning owners of animal subjects where the information collected relates primarily to the animal. This could include basic demographic information, information concerning animal routines and information concerning the animal’s history. Review of clinical data for the sole purpose of clinical audit does not require ethics approval if the intention is purely to improve clinical practice within the institution and with no aim of disseminating /publishing the results. If there is an intent to publish, it becomes clinical research and ethics approval is required.
- MVLS Ethics committee (Apply https://frontdoor.spa.gla.ac.uk/researchethics/Home.aspx) (Research involving human subjects or data)
Where the decision making, practices, and views of the animal owner (or other human) are the focus of the research rather than the research relating to the animal itself, this should be reviewed by the college of MVLS Ethics Committee, accessed via your staff portal: https://frontdoor.spa.gla.ac.uk/researchethics/Home.aspx.
There is often some overlap for questionnaire studies, so if unsure which committee to use please contact the SBOHVM Ethics Committee Convener, Joanna Morris.
Decision making flowchart for SBOHVM ethics approval
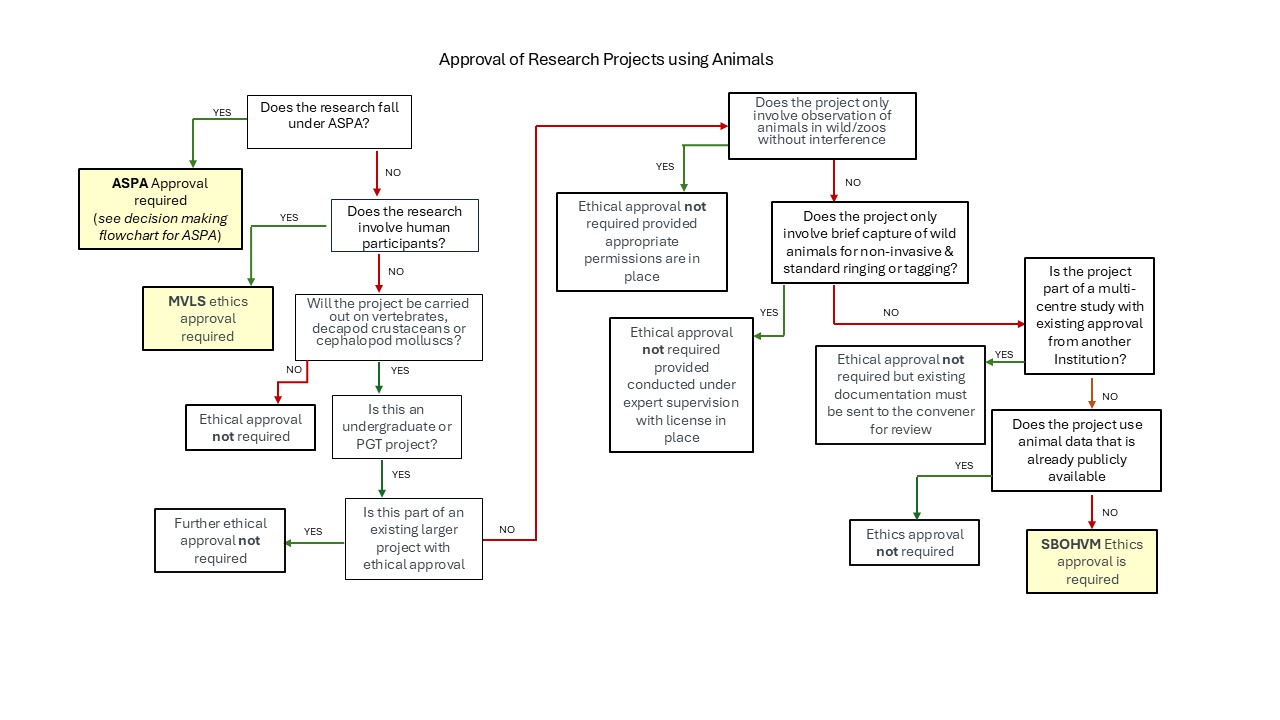
Animal related work which does not require SBOHVM approval
The following categories of animal work DO NOT require SBOHVM research ethics committee approval:
- Any project that falls under ASPA and needs HO licence – consult with Named Veterinary Surgeon
- Any project that involves invertebrates (EXCEPT decapod crustaceans or cephalopod molluscs)
- Any project that involves human subjects (questionnaires, focus groups etc) - would go through MVLS Ethics committee
- Undergraduate teaching material involving animal dissection etc
- Student work that piggybacks on ongoing animal research (which should already have permission in place from ASPA or SBOHVM)
- Any project that involves pure observation of wild animals in their natural surroundings with no disturbance /alteration of environment
- Any purely observational work at zoos/animal collections (however local permission would be needed) – seek advice if unsure.
- Any project that involves brief capture of wild animals/birds by standard measures eg mist nets purely for standard identification procedures, under expert supervision (e.g. bird ringing) and with appropriate licences
Animal related work which may need SBOHVM approval
The following categories of animal work may at least require a discussion about the need for approval/may need approval:
- Any study where wild vertebrates are brought into captivity, even if an observational study - see ASPA-Wild Animal Capture Policy document.
- Any study which involves housing animals specially for the work (we would need to check if housing is adequate)
- Any study where there are experimental interventions to housed vertebrates/ selected invertebrates that could compromise welfare e.g. a change in social grouping or diet or manipulation of environment
- Any projects which are purely breeding animals – seek advice
For many small trials of a very similar nature, it may be possible to consider a single application of longer duration to cover an area of work for a specific time period. However, it would need to be:
- Species specific
- Intervention specific
- Have a specified start and end date.
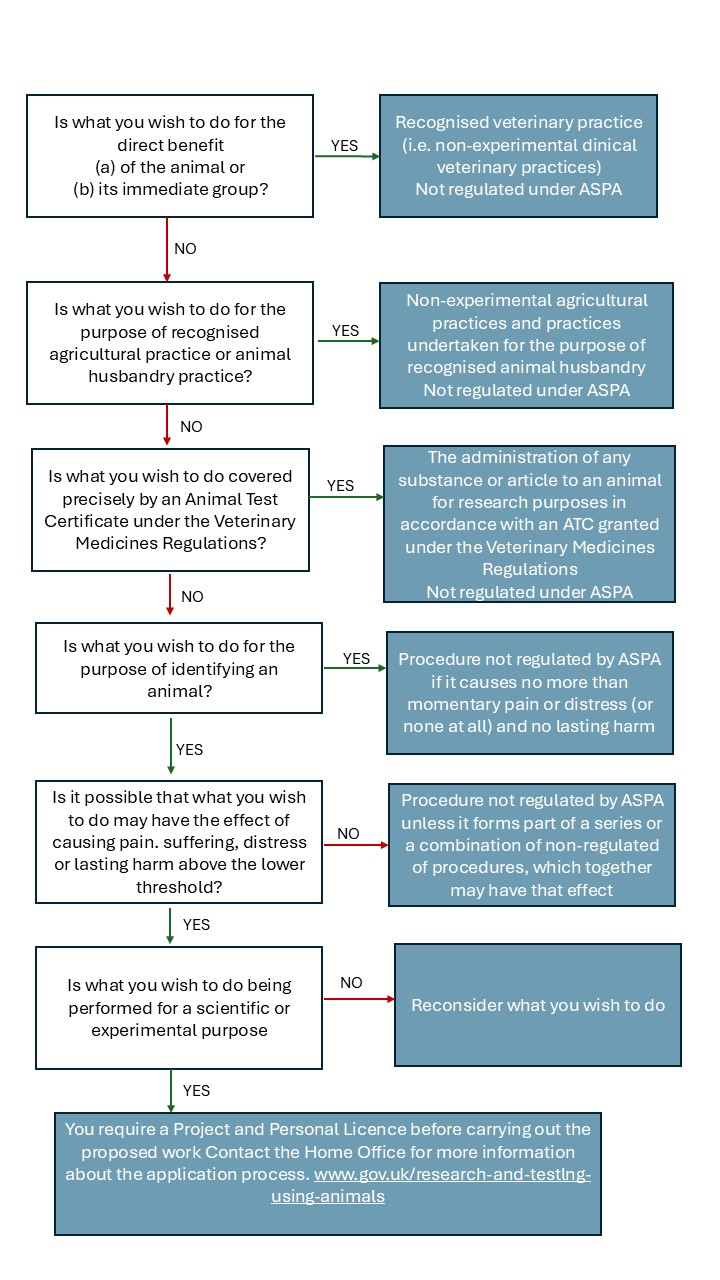
Veterinary clinical projects - decision making flowchart for ASPA
Guidelines on how to apply for Ethics approval through SBOHVM Research Ethics committee
Many journals require ethics approval as part of the submission process of research papers and it is important to seek this well in advance of starting any project that may result in publication. In addition, for field work that can only take place at certain times of the year, it is important to allow plenty of time to get approval prior to the work starting. Approval may take 4 weeks or more, to allow time for corrections/modifications requested by the committee. Retrospective approval for studies that have already been completed will not be granted.
Once the project outline is agreed and numbers of samples/animals have been estimated from a power calculation, complete the appropriate sections of the form, ensure all applicants have signed it and obtain Head of Division’s signature BEFORE submitting an electronic version to SBOHVM-ethics-requests@glasgow.ac.uk. Consent forms, owner information sheets and draft questionnaires (if relevant) should be submitted along with the form
Download the Research Ethics Application Form.
Retrospective studies involving clinical records or archived samples.
The RCVS Code of Professional Conduct clause 25.16 stipulates that Ethics review is required ‘when consent is needed for use of previously collected samples or the use of data from an animal’, therefore ethics approval should be sought even for projects that use archived samples, images or clinical data. For retrospective studies involving data from clinical records, archived images etc., only sections 1, 2, 4 and 8 of the form need to be completed.
Undergraduate and post-graduate teaching projects will be fast-tracked if the box on the front page of the form is ticked to indicate the project is for student teaching. Some projects may not require ethics approval at all, if they form part of a larger project that has been already approved, or if they are purely observational on animals in the wild or at a zoo, providing there is minimal disturbance of the animals and there is local approval.
Post-graduate research projects require the usual SBOHVM form to be completed.
Guidelines for form completion
The committee needs to be satisfied that the project has been well designed and that the use of animals is justified and does not subject them to unacceptable procedures. They will consider whether any samples being taken are collected non-invasively or are ethically justified e.g. are required for clinical purposes and only ‘left-over’ amounts will be used in research. Taking blood samples purely for research purposes and which are not for the clinical benefit of the patient (often a problem for control groups) is NOT permitted. Such sampling would fall under ASPA and would require authorisation via project and personal licences. There is no need to give exhaustive detail of the assays /analysis which will be used but it is important to see that the applicant is familiar with the literature and can justify the reason for the study, the number of cases for statistical power has been considered, that control groups have been selected appropriately and that the project is likely to provide valid results if it goes ahead. The applicant must consider how the animal subjects and or their owners are affected by the research project and what measures are in place to protect their interests.
Extra considerations
Consent forms
Although there are generic consent forms for clinical procedures at the Small Animal Hospital, Weipers Centre and Farm unit, which obtain consent for spare samples to be retained, any collection of non-invasive samples that are in addition to those needed for planned clinical purposes, would require a separate consent form eg. clipped hair, free catch urine, faeces etc. A client information sheet using text easily understandable by a lay person, briefly detailing the project and explaining the need for sample collection should accompany or be part of the consent form. The MVLS committee guidelines on participant information sheets for human subjects are comprehensive and can be adapted for animal projects.
It should be made clear to the participants what will happen to the data resulting from the study and how long it will be retained /shared with other researchers (university policy is 10 years).
Multi-centre studies
For collaborative projects with other centres where the other centre is the lead PI group, a copy of both the ethics application itself and the letter of approval should be submitted for approval by the SBOHVM convenor (not circulated to the committee) to ensure that samples provided from Glasgow are collected appropriately and GDPR is considered. For collaborations where the main PI is from University of Glasgow, it should be checked with collaborators whether additional ethics approval is needed at the other institutions involved, as well as at Glasgow. Consent forms at other institutions should comply with the requirement for specific consent for use of samples and data in research.
Animal test certificates (ATC)
Projects that are using veterinary medicinal products in a clinical/field setting to client owned animals require an ATC-type S. This may be unlicensed drugs or licensed drugs being used in a different way (off licence). Applications are submitted to the Veterinary Medicines Directorate at www.vmd.defra.gov.uk.
Project licences
For projects that are likely to come under ASPA both project and personal licences will be required as well as appropriate training. Advice should be sought from the named veterinary surgeon (NVS). Projects that would fall under ASPA in the UK but are being conducted in another country still require ethics approval through a sub-committee of the AWERB.
Risk Assessments
These will not be assessed by the committee but where relevant, it should be made clear that these have been applied for and/or granted and attached to the application.
Ethics or funding first?
To reduce work load we would not expect to review applications prior to funding unless the funder specifically requests this. However, ethical aspects of the work should be discussed with a committee member so that any issues may be identified. If necessary, a letter of comfort can be issued to accompany an application.
Amendments
For minor amendments to an application e.g. extension of project dates or no major changes to protocols, the convenor should be contacted directly.
For more substantial amendments, tracked changes on the initial application should be submitted and the project amendment form completed. Depending on the extent of the changes, the application may be circulated through the committee or dealt with by the convenor directly.

