Bioengineering breakthrough could improve bone regeneration treatments
Published: 7 June 2024
A bioengineering breakthrough which helps repair damaged bones without causing the negative side effects of other treatments could lead to better results for patients.
A bioengineering breakthrough which helps repair damaged bones without causing the negative side effects of other treatments could lead to better results for patients, scientists say.
It could support the development of new treatments to help people living with serious skeletal injuries or cancer patients who have lost bone to the disease to regrow bone tissue.
Researchers in Scotland have found a new way to harness the powerful healing effect of ‘growth factors’ – naturally-occurring molecules which help the body to regenerate.
Growth factors play an important role in developmental biology, helping to organise the development of bodies as they grow from infancy into adulthood. They also help the body to heal after injuries, where they initiate a complex series of processes which knit tissues back together.
Growth factor therapies, which use the targeted delivery of specific active proteins to encourage tissue regrowth, are promising methods for helping patients heal by boosting their body’s natural processes of regeneration.
However, growth factor therapies can have serious side-effects when used to heal bones. Active proteins need to be administered in high doses at the site of bone breaks or defects in order to be effective. Uncontrolled release of these active growth factors at the site of bone implant can cause bone formation where it is unintended – a process known as ectopic bone formation. The treatments can also cause other side effects like postoperative inflammation, which can have negative effects on patients’ health.
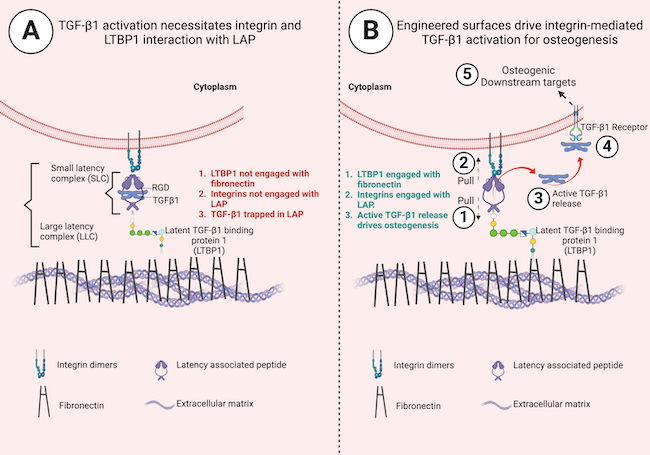
In a new paper published in the journal Advanced Materials, the University of Glasgow-led research team outline how they made their breakthrough. They used an inexpensive polymer called poly(ethyl acrylate), or PEA, to develop a surgical implant which can be used at the site of a bone defect. The unique properties of the implant’s surface allowed the team to capture the body’s inactive growth factors and ensure they start working only where they’re required.
Previous research from the team has shown that PEA interacts with fibronectin, a protein abundantly found in the human body which helps cells stick together and grow, to form nanoscale networks of the protein across its surface.
As the network forms, it changes the shape of the fibronectin, exposing some of the amino acids in the fibronectin molecule. Those amino acids are naturally used in the body to help cells attach as well as store inactive proteins.
The team dropped a recombinant protein fragment called latent transforming growth factor beta-binding protein-1, or rLTBP1, onto the fibronectin network, causing the two proteins to stick together.
rLTBP1 works like a magnet for a protein called TGF-β1, which encourages growth factor cells in the body to produce new bone tissue at low doses. TGF-β1 molecules are trapped in a protein complex called LAP, which keeps the protein’s ability to encourage bone regeneration inactive until it is required.
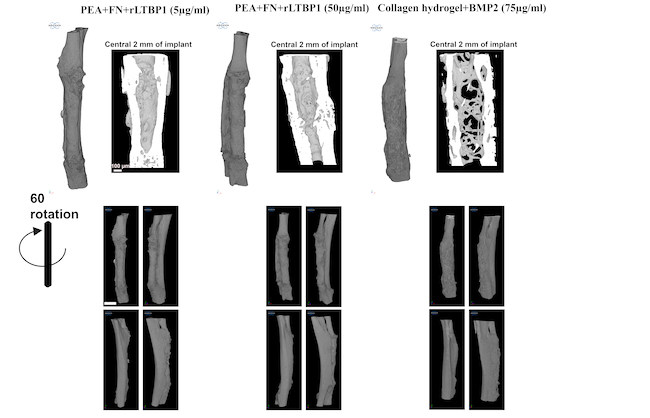
They coated small plastic tubes with PEA, fibronectin and rLTBP1. Then, they demonstrated the potential of these implants to regenerate bone in critical-sized defect in mice. Over the course of the study, they observed complete regeneration of bone defect.
When bone-regenerating cells arrived at the site of bone defect, attachment sites on the cells’ surface called integrins latched onto the LAP molecules that the rLTBP1 on the implant surface had immobilized. Through mechanical pulling of LAP on one side by integrins and on the other by rLTBP1, TGF-β1 was released, which kickstarted its growth factor signaling processes. This resulted in controlled bone formation only where it was required.
Dr Udesh Dhawan, Research Fellow at the University of Glasgow’s James Watt School of Engineering, is the paper’s lead author. He said: “The biological processes that underpin this study have been understood for more than two decades, but this is the first time that they’ve been harnessed to produce this regenerative effect.
“Being able to deliver immobilised proteins directly to the treatment site in this way provides much more control over how growth factors become active and start the healing process. It also works at much lower concentrations than previous treatments, helping further minimise the chances of unwanted bone growth beyond the site in need of healing.”
The team’s findings build on years of advanced bone regeneration research led by the University of Glasgow’s Professor Manuel-Salmeron-Sanchez and Professor Matthew Dalby. Their work includes a landmark treatment which saved a badly injured dog’s leg from amputation in 2017.
In that treatment, the team harnessed the growth factor potential of a protein called BMP-2 bonded to a PEA surface to regrow bone in the dog’s leg. In the new paper, the team show how they used BMP-2 in a parallel control study to help measure the effectiveness of rLTBP1 in regenerating bone. They found that rLTBP1 worked just as well as BMP-2 in helping to regenerate bones, even though it was administered in lower doses.
Professor Salmeron-Sanchez, co-director of the University of Glasgow’s Centre for the Cellular Microenvironment, said: “Growth factors are very powerful tools for helping the body heal, but currently they need to be very carefully applied to prevent negative side effects cancelling out any positive therapeutic benefits. Our approach to controlling the activation of growth factors could create new opportunities for patients in the future. It could help regrow bone for patients who have lost large sections to diseases like cancer or through serious accidents, providing a much higher quality of life for them.”
Dr Dhawan added: “This is a new step in the right direction, but physiological systems are more interconnected than we can imagine and how this new strategy affects other crucial components of the body such as immune cells still needs to be evaluated. Nevertheless, these are very encouraging results, which suggest that this new treatment could have real benefits in clinical settings to encourage bone regeneration.”
The team’s paper, titled ‘Engineered Surfaces That Promote Capture of Latent Proteins to Facilitate Integrin-Mediated Mechanical Activation of Growth Factors’, is published in Advanced Materials.
The research was supported by funding from the European Union’s Horizon 2020 programme, the European Research Council, and the Engineering and Physical Sciences Research Council.
First published: 7 June 2024
<< June

